What is hereditary hemochromatosis?
Hereditary hemochromatosis is a genetic disorder characterized by increased iron absorption. The increased iron absorption leads to the accumulation of iron stores in multiple organs, resulting in cirrhosis, diabetes, cardiomyopathy, or hypogonadism. Hereditary hemochromatosis is categorized into four types: type 1 hemochromatosis (HFE-related), type 2 (A and B) hemochromatosis (juvenile hemochromatosis), type 3 hemochromatosis (transferrin receptor 2 hemochromatosis), type 4 (A and B) hemochromatosis (ferroportin disease) (Brissot et. al., 2008). The focus of this project is hereditary hemochromatosis type 1 as it is the most common form of the disease.
How common is it?
In general, the disease has a high incidence in those of northern European descent, with an estimated prevalence of between 1 in 200 and 1 in 500 individuals. In the United States, hemochromatosis is seen in 1 in 300 non-Hispanic whites, with even lower rates among other ethnicities and races, affecting about 1 million people (Bacon et. al., 2011).
How is it diagnosed?
People with a family history of the disease are more prone to acquire it themselves. A variety of tests can screen for the presence of the disease. Blood tests of serum transferrin and hepcidin levels can be measured to detect the levels of iron present. MRI scans can detect iron accumulation in the organs. Genetic testing for the specific mutations of the disease can further verify the diagnosis for hemochromatosis.
What are the symptoms?
The most common symptoms are fatigue, joint pain, abdominal pain, diabetes, and cirrhosis (Bacon et. al., 2011). The broad set of symptoms can cause difficulty in diagnosing the disease, which is why early diagnosis is crucial for proper treatment. Furthermore, up to 18% of men and 5% of women can have hepatic iron overload in the absence of clinical symptoms (Kowdley et. al., 2019). The disease is seen more often in men than in women. Iron accumulation is slower in women due to the blood loss through menstruation (Kowdley et. al., 2019). Hemochromatosis is present at birth, but symptoms tend to manifest later in life.
What causes it?
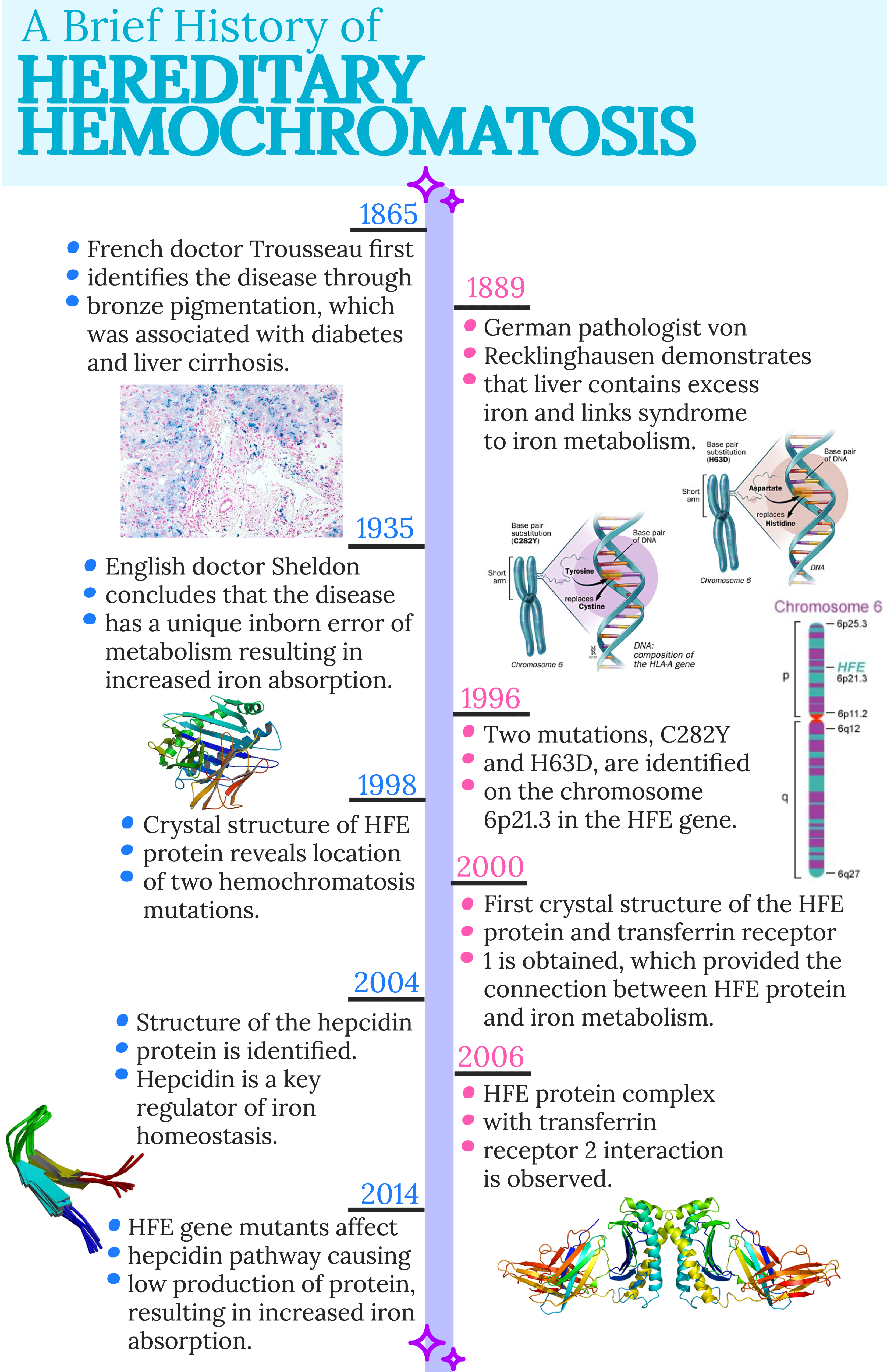
Hemochromatosis is due to mutations on chromosome 6 in the HFE gene, which encodes for the HFE protein. The two most common mutations are C282Y and H63D. The C282Y is a single point mutation with a substitution at the 282 position of amino acid tyrosine for a cysteine. Also a single point mutation, the H63D mutation is a substitution at the 63 position of aspartate for histidine.
The HFE protein binds to the transferrin receptor, which causes the iron-bound transferrin affinity to decrease. Normally, the HFE protein plays a role in regulating the hepcidin protein, which is responsible for controlling iron transport into the blood. Hepcidin ubiquitinates and degrades ferroportin, which is a protein that transports iron into cells. A zoomed out view of iron absorption is depicted in Figure 1.

Abbreviations: DMT1, divalent metal transporter 1.
The expression of hepcidin is controlled via a signal transduction pathway that occurs once the HFE protein is bound to the transferrin receptor. However, the C282Y and H63D mutations of hemochromatosis disrupt the normal function of the signal transduction pathway, resulting in decreased hepcidin expression (Wu et. al., 2014). Since the expression of hepcidin is downregulated, ferroportin is not degraded and iron is continuously transported into the cells and accumulates inside, resulting in iron overload.
What are the treatments?
Early treatment of hemochromatosis can prevent permanent organ damage. The primary targets of the disease are hepatic function, glucose tolerance, cardiac function, thyroid function, and gonadal function (Kawabata et. al., 2018). Consequently, diabetes, cardiomyopathy, hypothyroidism, and hypogonadism can result. Targeting these issues is essential in attempting to treat hemochromatosis.
The most common and effective treatment is phlebotomy, which is the surgical opening of a vein for blood removal. For people who are unable to undergo phlebotomy treatments, iron chelation drugs can be administered. Although the drugs show high levels of iron depletion, the side effects are toxic and can lead to additional liver damage from excess usage.
The increased iron absorption can be targeted by proton pump inhibitors, which inhibit a specific protein that is essential for iron uptake. Additionally, dietary changes can decrease the effects of iron overload. Specifically, consumption of red meat, limitation of vitamin C, and no consumption of raw shellfish are examples of ways to decrease the rate of iron accumulation (Adams et. al., 2010).

Created by Adobe Spark.
For more information on hereditary hemochromatosis, please visit the following pages.
Normal Function of Key Players
Identification and Characterization
Effects, Detection, and Treatments
References
Adams, P. C.; Barton, J. C. How I Treat Hemochromatosis. Blood 2010, 116 (3), 317–325.
Bacon, B. R.; Adams, P. C.; Kowdley, K. V.; Powell, L. W.; Tavill, A. S. Diagnosis and Management of Hemochromatosis: 2011 Practice Guideline by the American Association for the Study of Liver Diseases. Hepatology 2011, 54 (1), 328–343.
Brissot, P.; Troadec, M.-B.; Bardou-Jacquet, E.; Lan, C. L.; Jouanolle, A.-M.; Deugnier, Y.; Loréal, O. Current Approach to Hemochromatosis. Blood Reviews 2008, 22 (4), 195–210.
Kawabata, H. The Mechanisms of Systemic Iron Homeostasis and Etiology, Diagnosis, and Treatment of Hereditary Hemochromatosis. International Journal of Hematology 2018, 107 (1), 31–43.
Kowdley, K. V.; Brown, K. E.; Ahn, J.; Sundaram, V. ACG Clinical Guideline: Hereditary Hemochromatosis. American Journal of Gastroenterology 2019, 114 (8), 1202–1218.
Sankaran, V. G.; Weiss, M. J. Anemia: Progress in Molecular Mechanisms and Therapies. Nature Medicine 2015, 21 (3), 221–230.
Wu, X.; Wang, Y.; Wu, Q.; Cheng, W.-H.; Liu, W.; Zhao, Y.; Mayeur, C.; Schmidt, P. J.; Yu, P. B.; Wang, F.; Xia, Y. HFE Interacts with the BMP Type I Receptor ALK3 to Regulate Hepcidin Expression. Blood 2014, 124 (8), 1335–1343.

10 Responses
How does female menstruation help to reduce the number of cases in females versus males?
Thanks for your comment, Lindsay! So, the whole basis of this disease is iron overload in the body. That being said, given the mutations that cause the iron overload to occur, the excess iron has to actually stay in the body in order to eventually accumulate. Iron is expelled through the blood loss of female menstruation, so there is always the possibility of the excess iron that results from hereditary hemochromatosis being included in that loss. Since the iron is lost, the rate of accumulation is not that high, and the number of cases of hereditary hemochromatosis is low in women. However, after menopause, the number of cases in women does increase, since that monthly blood loss is no longer occurring.
Being that the most effective treatment of hemochromatosis is phlebotomy, how often does this need to be administered? Is this a process that needs to be done regularly?
Thanks for your comment, Nick! Phlebotomy is the most effective treatment for this disease. The standard volume that is removed from a patient is 400-500 mL, although the amount is modified based on age, body weight, and hemoglobin levels. In order for the treatment to provide the most effective results, it is typically administered once a week until iron levels have decreased to normal levels, which can take several months or years. For more information on other therapies, feel free to check out my page on treatments!
Is there a connection between exercise and iron accumulation?
Thanks for your question, Jishan! Generally, the mutation that causes hemochromatosis actually has positive and negative effects on the body. In terms of exercise, an iron overload can result in lethargy, chronic fatigue, and weakness in a person who does not typically exercise. However, if a person with hereditary hemochromatosis regularly exercises, the mutations can result in increased iron supply during physical activity, which ultimately aids in height and life span. You can check out more information on the effects of the disease on my pages here!
If you are diagnosed with the disease, how likely are you to pass it on to your children? Are there any other factors that can decrease the chances of passing it on?
Thanks for your questions, Nicole!! Hemochromatosis is a genetic disorder that is inherited in an autosomal recessive manner, which means that two copies of the mutated gene must be present in order for the disease to develop. In terms of being passed onto children, it depends on whether or not both parents have the disease. If both parents have the disease, then there is a higher chance of the children inheriting the disease. However, if only one parent has the disease, then the likelihood of it being passed on decreases. For more detail on the mutations, you can check out this page!
There is really no direct way of decreasing the chances of passing the disease on to future generations. However, identifying the disease in its early stages is key to prevent permanent damage later on in life. Feel free to check out my page on ways to detect the disease!
This is so informative! What is the criteria for someone to get the phlebotomy surgery?
Thanks for your comment, Jess! The phlebotomy treatment is actually very similar to a blood test procedure, in which blood is taken from a vein on the back of the hand or below the elbow. As the blood is drawn, the blood pressure is monitored during the procedure. This therapeutic use of phlebotomy removes a larger amount of blood than a normal blood donation.
The goal of the phlebotomy treatment is to lower iron levels to normal. The amount of blood that is removed depends on the age, overall health, and the severity of iron overload of a patient. Based on these characteristics, phlebotomy can be administered and used to decrease iron levels. However, this treatment can take several months or years, depending on the amount of blood that is removed. There are more treatments available for hemochromatosis patients. Feel free to get more information on some of those treatments here!